Stem cell organization dictates the impact of proliferation rate on Biology Diagrams Growing evidence indicates that this machinery operates in a distinct fashion in some mammalian stem cell types, such as pluripotent embryonic stem cells. The cell cycle in stem cell proliferation, pluripotency and differentiation Nat Cell Biol. 2019 Sep;21(9):1060-1067. doi: 10.1038/s41556-019-0384-4. The mitochondrial pyruvate carrier MPC1 is dynamically regulated in neural stem cells (NSCs), and in vitro and in vivo genetic ablation of MPC1 leads to NSC activation. 55 Similarly, deletion of MPC1 in intestinal stem cells (ISCs) leads to increased stem cell proliferation in vivo and in vitro organoid formation. 56 In hair follicle stem cells Stem cells persist throughout the lifespan to repair and regenerate tissues due to their unique ability to self-renew and differentiate. Here we reflect on the recent discoveries in stem cells that highlight a mitochondrial metabolic checkpoint at the restriction point of the stem cell cycle. Mitochondrial activation supports stem cell proliferation and differentiation by providing energy

Summary. Human embryonic stem (hES) cells show an atypical cell cycle regulation characterized by a high proliferation rate and a short G1-phase [1, 2].In fact, a shortened G1-phase might protect ES cells from external signals inducing differentiation, as shown for certain stem cells [].It has been suggested that self-renewal and pluripotency are intimately linked to cell cycle regulation in

Translational control of stem cell function Biology Diagrams
Stem cells are well known to be controlled transcriptionally, but recent studies indicate that pluripotency, cell fate and differentiation depend on the regulation of translation and ribosome
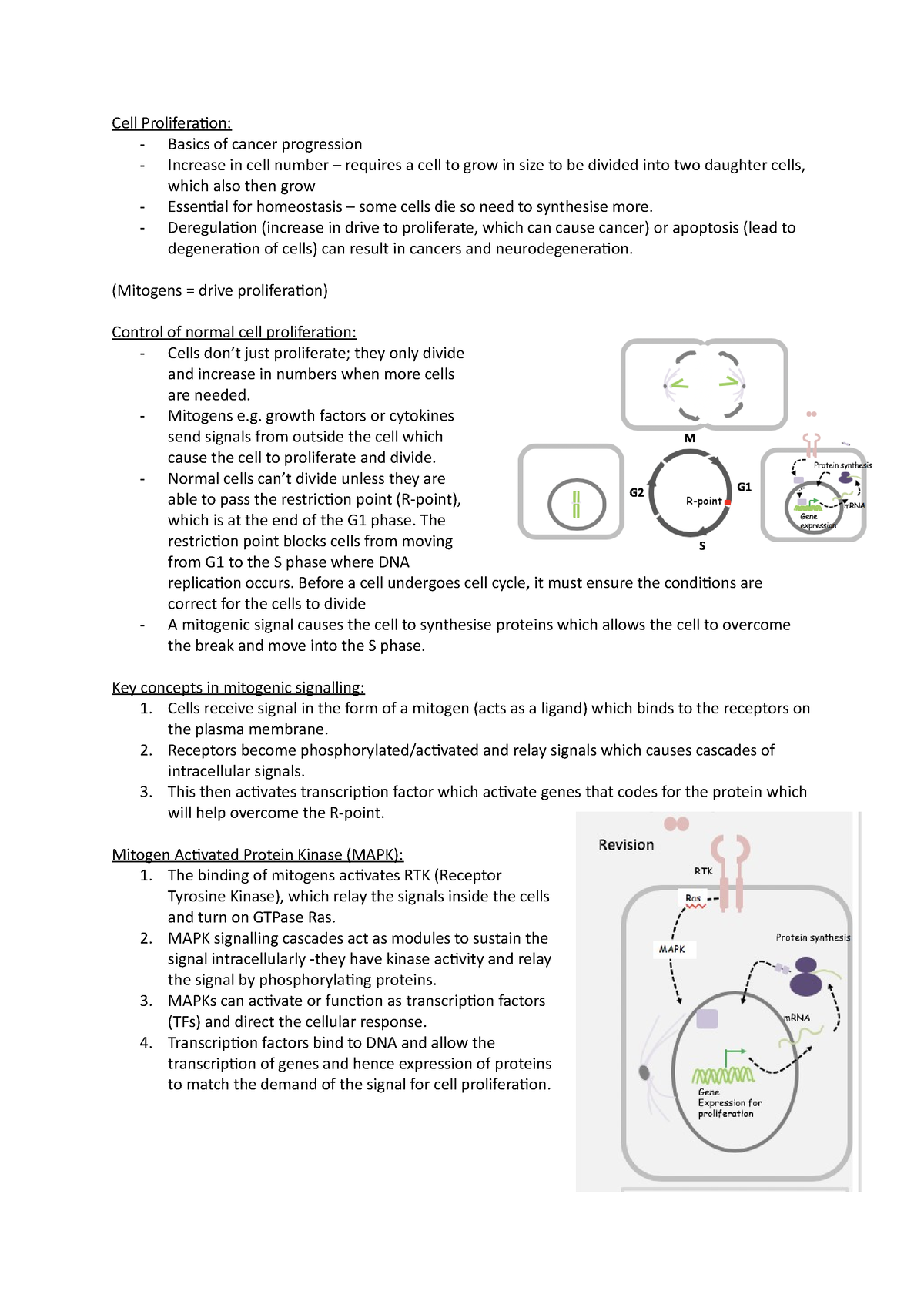
In this review, we discuss our current knowledge of how cell cycle proteins mechanistically link cell proliferation, pluripotency and cell fate specification. We focus on embryonic stem cells, induced pluripotent stem cells, and embryonic neural stem/progenitor cells. Cheung TH & Rando TA Molecular regulation of stem cell quiescence. Nat Transcription is known to tightly regulate gene expression profiles in stem and differentiating cells, but less is known about the role of translational control. This study identifies that signals from the stem cell niche induce changes in translational rates, regulated through eIF3d1 phosphorylation, to control stem cell fate in a Drosophila testis model. In direct conversion, cells do not necessarily transit through a defined progenitor state, 9, 37 and proliferation is not required for conversion. 38, 39, 40 However, highly proliferative cells reprogram at higher rates to both induced pluripotent stem cells (iPSCs) and post-mitotic neurons, suggesting that proliferation may serve a conserved

